Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
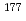 Lu via the
Lu via the 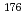 Yb(n,
Yb(n, )
)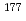 Yb
Yb
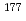 Lu process
Lu processHashimoto, Kazuyuki; Matsuoka, Hiromitsu; Uchida, Shoji*
Journal of Radioanalytical and Nuclear Chemistry, 255(3), p.575 - 579, 2003/03
Times Cited Count:42 Percentile:91.74(Chemistry, Analytical)The 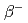 emitter
emitter 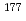 Lu is a promising therapeutic radioisotope for the treatment of cancer. It has a half-life of 6.73 days and maximum
Lu is a promising therapeutic radioisotope for the treatment of cancer. It has a half-life of 6.73 days and maximum 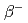 energy of 498 keV, resulting in a short range of radiation in tissue. The decay is accompanied by the emission of low energy
energy of 498 keV, resulting in a short range of radiation in tissue. The decay is accompanied by the emission of low energy  -radiation with
-radiation with 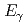 = 208 keV (11.0%) and 113 keV (6.4%) suitable for simultaneous imaging. Lutetium-177 can be usually produced at nuclear reactors with high yield and high specific radioactivity by the
= 208 keV (11.0%) and 113 keV (6.4%) suitable for simultaneous imaging. Lutetium-177 can be usually produced at nuclear reactors with high yield and high specific radioactivity by the 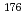 Lu(n,
Lu(n, )
)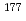 Lu reaction. However, radioisotopes with higher specific radioactivity are required in the field of radioimmunotherapy using labeled monoclonal antibodies. Thus, an alternative production route, namely the
Lu reaction. However, radioisotopes with higher specific radioactivity are required in the field of radioimmunotherapy using labeled monoclonal antibodies. Thus, an alternative production route, namely the 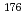 Yb(n,
Yb(n, )
)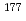 Yb
Yb 
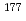 Lu process was studied to produce no-carrier-added (nca)
Lu process was studied to produce no-carrier-added (nca) 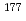 Lu in this work. The radiochemical separation of the nca
Lu in this work. The radiochemical separation of the nca 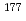 Lu from the macroscopic ytterbium target was investigated by means of reversed-phase ion-pair HPLC. The nca
Lu from the macroscopic ytterbium target was investigated by means of reversed-phase ion-pair HPLC. The nca 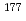 Lu was obtained in radiochemical pure form with a separation yield of 80%.
Lu was obtained in radiochemical pure form with a separation yield of 80%.
Naganawa, Hirochika; Suzuki, Hideya; Tachimori, Shoichi; Nasu, Akinobu*; Sekine, Tatsuya*
Physical Chemistry Chemical Physics, 3(12), p.2509 - 2517, 2001/06
Times Cited Count:34 Percentile:71.56(Chemistry, Physical)no abstracts in English
Naganawa, Hirochika; Suzuki, Hideya; Tachimori, Shoichi; Nasu, Akinobu*; Sekine, Tatsuya*
Bulletin of the Chemical Society of Japan, 73(3), p.623 - 630, 2000/03
Times Cited Count:6 Percentile:35.29(Chemistry, Multidisciplinary)no abstracts in English
 -diketone in synergistic extraction of actinide(III) and lanthanide(III) with
-diketone in synergistic extraction of actinide(III) and lanthanide(III) with  -diketone+18-crown-6 ether/1,2-dichloroethane
-diketone+18-crown-6 ether/1,2-dichloroethaneMeguro, Yoshihiro; Kitatsuji, Yoshihiro; Kimura, Takaumi; Yoshida, Zenko
Journal of Alloys and Compounds, 271-273, p.790 - 793, 1998/00
Times Cited Count:21 Percentile:73.86(Chemistry, Physical)no abstracts in English
; *; Tachimori, Shoichi
Bulletin of the Chemical Society of Japan, 69(10), p.2869 - 2875, 1996/00
Times Cited Count:3 Percentile:27.55(Chemistry, Multidisciplinary)no abstracts in English
Kitatsuji, Yoshihiro; Meguro, Yoshihiro; Yoshida, Zenko; *; *
Solvent Extr. Ion Exch., 13(2), p.289 - 300, 1995/00
Times Cited Count:64 Percentile:90.82(Chemistry, Multidisciplinary)no abstracts in English
; Tachimori, Shoichi
Bulletin of the Chemical Society of Japan, 67, p.2690 - 2699, 1994/00
Times Cited Count:7 Percentile:43.92(Chemistry, Multidisciplinary)no abstracts in English
Meguro, Yoshihiro; Yoshida, Zenko
Radiochimica Acta, 65, p.19 - 22, 1994/00
no abstracts in English
Shitsuryo Bunseki, 27(4), p.217 - 245, 1979/00
no abstracts in English
Kinoshita, Ryoma; Sasaki, Yuji; Kaneko, Masashi; Matsumiya, Masahiko*; Shinoku, Kota*; Shiroishi, Hidenobu*
no journal, ,
Many metal ions are stable in hydrochloric acid solutions as anionic species; diamidic-extractants containing tertiary amino nitrogen atom, such as NTAamide (hexaalkyl-nitrilotriacetamide), are normally protonated in acidic solutions and become cationic extractants. In this study, the ion-pair extraction reactions of metal chloride anions with cationic NTAamide(C6) extractant were investigated. In addition, we attempted to predict the distribution ratio by combining the stability constants of metal chloride complexes and DFT calculations, and compared the results with experimental values.
Sasaki, Yuji; Ban, Yasutoshi; Matsumiya, Masahiko*
no journal, ,
Metal ions are present as anionic species in hydrochloric acid. Diamino-extractants containing tertiary amino N atom, such as NTAamide, protonate in acidic solutions and become cationic extractants. In this study, the ion-pair extraction of metal chloride anions, especially platinum metals and the related metals, with the cationic extractants was performed and their extraction properties are studied.